The RTS,S malaria vaccine trial …… a take home message
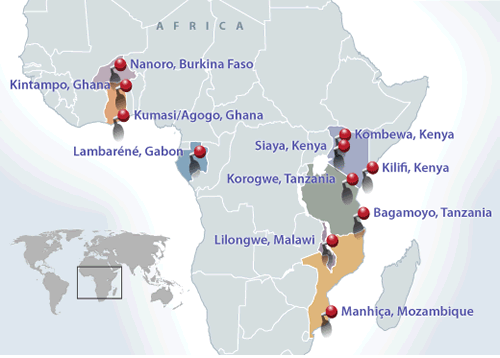
The world has been eagerly awaiting the definitive clinical trial on the malaria vaccine known as RTS,S. The results were published in The Lancet journal on the 24th April 2015. This trial was a biggie – a total of 15,459 children were recruited. These children were from 11 sites in 7 countries across Africa.
Kenya contributed 3 of those sites: Siaya, Kilifi and Kombewa (Kisumu).
The clinical trials in Kenya were led by Dr Patricia Njuguna of the KEMRI-Wellcome Trust Research Program in Kilifi. In fact Dr Njuguna was the only female lead investigator in the all 11 study sites.
Recruitment into the study begun in 2009. There were two groups of children used in the study: those 5-17 months and those 6-12 weeks. The scientists wanted to know in which age group the vaccine worked best. The ideal group would be the 6-12 week children who are already getting the Expanded Program for immunisation (EPI) vaccines. These children receive 3 doses of DPT and PCV10 vaccine and this schedule would tie in nicely with the schedule for the malaria RTS,S vaccine which also required a child to receive 3 doses of the vaccine a month apart. I would imagine that the scientists were hoping the vaccine would work in this age group and that the RTS,S vaccine would introduced as part of the EPI program.
After a year of follow-up the results of this trial were published in 2011. The results showed that the vaccine reduced malaria episodes by half in children 5-17 months and by a third in children 6-12 weeks. There appeared to be some hope in the vaccine. However, earlier studies on the same vaccine conducted by a Tanzanian researcher, Dr Ally Olutu had shown that immunity to the RTS,S malaria vaccine waned over time.
Due to the results produced by Dr Olutu, the researchers decided to introduce a booster vaccination of the RTS,S malaria after 18 months of the initial dose. They also decided to observe the children for between 3 to 4 years in order to see whether protection from the vaccine waned with time.
So what did the results show?
Among children aged 5-17 months, the vaccine offered 36 percent protection against outpatient malaria and 32 percent against severe malaria requiring hospitalisation, among those who received the booster vaccination. Among infants 6-12 weeks vaccinated according to the EPI schedule, the vaccine offered 26 percent protection against outpatient malaria and according to the statistics, no protection against severe malaria requiring hospitalisation among those who received the booster vaccination. The protection was lower among those children that did not receive booster vaccinations.
For a vaccine to offer a protection of 36-26% is not impressive at all. Worse was that it showed the poorest protection in those children 6-12 weeks who would have been the best targets for the vaccine. In these tiny tots, the vaccine did not protect against malaria severe enough for a child to be admitted into hospital.
For a malaria vaccine to have an impact, it must show protection against malaria severe enough for a child to be admitted or malaria deaths. The vaccine did not protect against malaria mortality in all age groups. However, there is a caveat here, there were very few severe malaria cases and very few malaria deaths during the period of the trial. This made it difficult to show protection against these two outcomes. Perhaps, and this is a perhaps, the vaccine might show protection against these two outcomes in field conditions.
But this result highlights a very useful aspect of malaria control. During the study, parents and caregivers were advised to take their child to see a clinician any time they had a fever and this ensured that all malaria cases were treated on time. This is what enabled the severe malaria cases and deaths to be brought down during the study period. Imagine this for a moment. A total of over 15,000 children were being observed closely with parents encouraged to receive quality care and that this was enough to bring hospitalisations and deaths down to a point were it was impossible to compare the impact of the vaccine. It illustrates just how important it is for malaria to be treated on time.
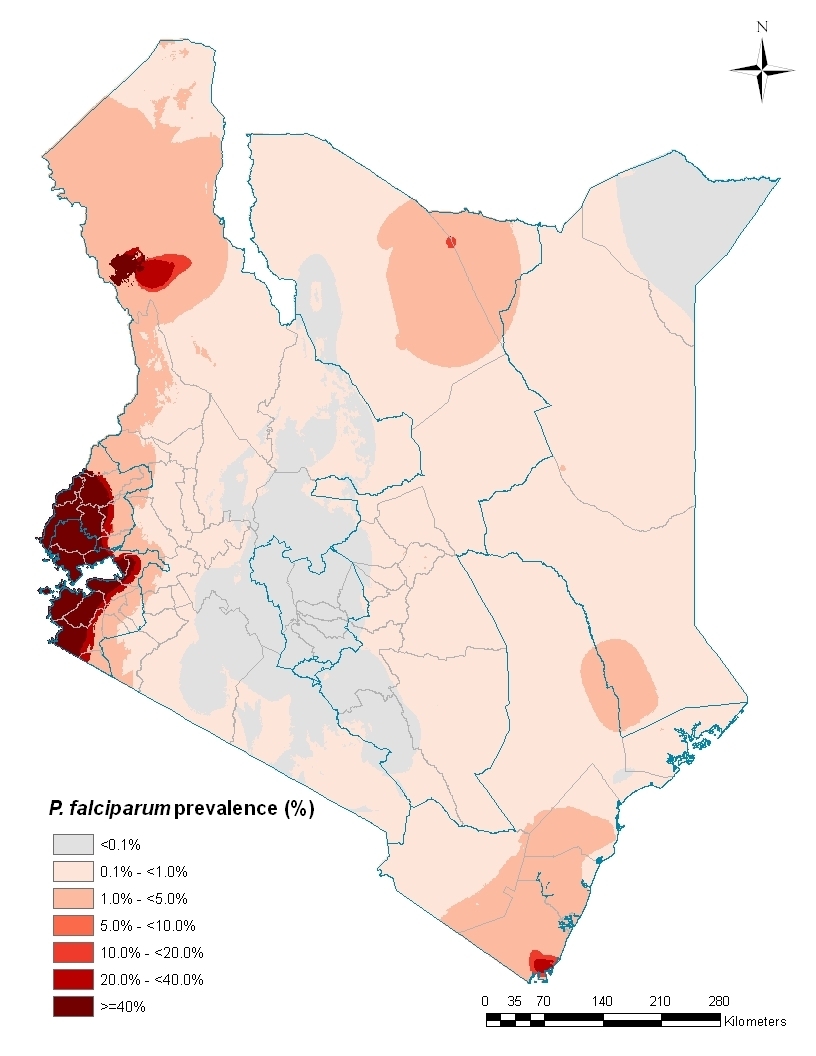
The paper published in the Lancet for World Malaria Day – 25th April – made a lot about cases of malaria illness averted by the vaccine. Of interest are the results in the 6-12 week group of children. The number of cases of malaria averted were calculated per 1,000 children who received the vaccine (vaccinees). In Kilifi, which was the site with the lowest malaria transmission of all 11 sites, only 20 clinical malaria cases were averted per 1,000 vaccinees, however, in high transmission areas like Siaya, close to 3,500 cases were averted per 1,000 vaccinees. However, for malaria severe enough to require hospitalisation, the RTS,S vaccine averted only under 60 cases per 1,000 vaccinees in Siaya.
A lot of outpatient malaria was averted in high transmission areas like Siaya, however the vaccine appeared to be of hardly any use in areas of low malaria transmission, like Kilifi, where very few cases were averted.
If the government choose to use the vaccine, for it to be cost effective, it would have to restrict the use to the high transmission areas in Kenya. According to the malaria risk map, this would be only around Lake Victoria.
The study did however show that if the government focused on ensuring that all children with fever in malaria endemic areas are treated on time, with the highly effective Co-artem or any artemesinin derivative, then the country would control the number of severe cases and deaths. To focus on this along with provision of treated bednets and house spraying would avert a lot more malaria cases than funneling resources into provision of this vaccine, whose protection even in this controlled trial environment was extremely poor.
Looking at the evidence provided in this paper, from a thorough piece of research – the Kenyan government should not consider using the malaria vaccine as part of its malaria control efforts.
However, Prof Adrian Hill of the University of Oxford is working to improve on RTS,S by adding another antigen ME-TRAP (please don’t ask me the details of this yet!) into the mix. The trials for this modified RTS,S may show results that may be encouraging – we should wait and see.
The results of this vaccine trial are a disappointment considering the work that has gone into it : decades of work and millions of dollars, the various scientists and children that have been involved. It has however, build capacity for African institutes and scientists to run clinical trials at the highest level – it has created systems that will be useful when finally a vaccine with a higher efficacy comes along. Dispensaries in remote regions that participated in the study have benefited from this exposure and the money that went to developing the infrastructure of these facilities.
I am however convinced from the evidence that this vaccine is of no use as it stands now. It requires a lot of effort for little output – a cost-effectiveness analysis is under way which may shed more light into this and may prove me wrong. People will say that RTS,S averts thousands of cases – but providing insecticide treated bednets to everyone and treating all clinical malaria episodes on time will actually bring us closer to malaria control than this vaccine in it’s current form ever will.
Comments
-
Mary
I look forward to the details/outcome of the cost-effectiveness analysis bit, since its a key concept in Health Economics and Health systems management as a whole.
Comments are closed.




Mary
Great insights.